PRS Technology
Selecting the appropriate counter-ion for an ion-exchange membrane depends on the research objective. In general, counter-ions should have low affinity for the ion-exchange membrane, thus allowing nutrient ions in the soil solution to be readily adsorbed. Commonly, H+ and OH- are used as counter-ions for cation and anion-exchange membranes, respectively. However, these counter-ions are inappropriate for many soil applications. For example, in calcareous soils the H+ reacts with abundant CaCO3 and liberates Ca2+. This large quantity of free Ca2+ interferes with NH4+ and K+ adsorption and will affect NH4+ and K+ supply rate measurements if the ion-exchange membrane is left in soil for a long period of time. Furthermore, the addition of H+ and OH- may introduce undesirable rhizosphere effects (i.e., pH), which will affect nutrient availability.
Cation
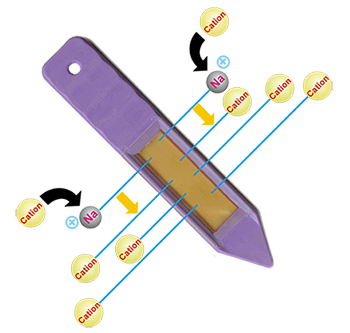
Cation PRS® Probes use Na+ as the counter-ion. The relative affinity of Na+ is the next lowest in order to H+, but its use avoids undesirable rhizosphere effects (i.e., reduced pH).
Anion
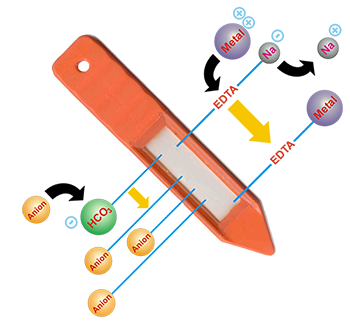
Anion PRS® Probes use HCO3- as the primary counter-ion, but also use ethylenediaminetetraacetate (EDTA) as the counter-ion on approximately 30% of the ion-exchange sites. The HCO3- is used due to its low affinity and because desorbed HCO3- bio-mimics the HCO3- produced in the rhizosphere during plant root and microbial respiration. The HCO3- is also not conserved in the solution, thereby helping to overcome the complementary anion effect, which may reduce adsorption of soil anions. The EDTA is used to increase adsorption of P and micronutrients, in particular polyvalent metal cations such as Al, Fe, Mn, Cu, and Zn.
